AbbVie Receives Positive Recommendation from the pan-Canadian Oncology Drug Review Expert Review Committee for the Combination VENCLEXTA® With Rituximab as a Treatment for Patients With Chronic Lymphocytic Leukemia
- pERC conditionally recommends reimbursement of VENCLEXTA® (venetoclax) in combination with rituximab for the treatment of adult patients with chronic lymphocytic leukemia (CLL) who have received at least one prior therapy.i
- Adult patients with CLL taking VENCLEXTA in combination with rituximab can stop their therapy after a defined treatment period of 24 months on treatment.
MONTREAL, June 6, 2019 /CNW/ - AbbVie (NYSE: ABBV), a global research and development-based biopharmaceutical company, today announced that the pan-Canadian Oncology Drug Review (pCODR) Expert Review Committee (pERC) conditionally recommends reimbursement of VENCLEXTA® (venetoclax) in combination with rituximab for the treatment of adult patients with chronic lymphocytic leukemia (CLL) who have received at least one prior therapy, irrespective of their 17p deletion status, only if the following condition is met: cost-effectiveness being improved to an acceptable level.i VENCLEXTA in combination with rituximab is an effective treatment option that has the benefit of a finite treatment approach, meaning patients can able to stop their therapy after two years of treatment.
"With venetoclax plus rituximab, patients receive a highly effective treatment that leads to durable remission while having a clear defined end-date. The concept of finite treatment duration is something my patients appreciate because they have the ability to be off therapy," says Dr. Carolyn Owen, MD, MDres(UK), FRCPC, a Hematologist and associate professor at the University of Calgary.
pERC concluded in its report that VENCLEXTA plus rituximab aligns with patient values in that it provides additional treatment choice, delays disease progression, has manageable side effects, a finite treatment duration, and a partially oral route of administration.i
"The pCODR recommendation for VENCLEXTA plus rituximab is positive news for Canadians living with CLL," says Elizabeth Lye, Director of Research & Programs, Lymphoma Canada. "Receiving a diagnosis of CLL or any cancer is always shocking and overwhelming, therefore knowing that there are highly effective treatments available provides reassurance to people facing this uncertain journey."
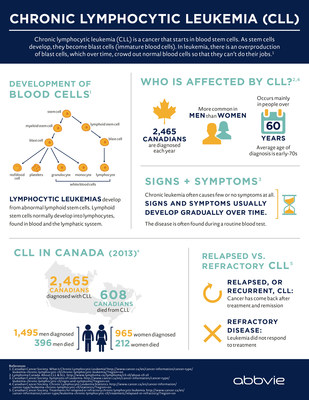
CLL, which is typically a slow-progressing cancer of the bone marrow and bloodii, is one of the most common types of leukemia in adults. In Canada, CLL accounts for approximately 2,465 newly diagnosed cases of leukemia each year and is responsible for more than 600 deaths a year.iii The goal of treatment is to delay progression of the disease and improve quality of life.
"This is another tremendous milestone in our efforts to bring VENCLEXTA plus rituximab to Canadians living with CLL. This is a much needed treatment option as it is the first chemotherapy-free combination in CLL that allows patients a 24-month treatment duration," says Stéphane Lassignardie, General Manager of Abbvie Canada.
VENCLEXTA continues to be investigated in CLL and other hematological diseases.
VENCLEXTA is being developed by AbbVie and Roche. It is jointly commercialized by AbbVie and Genentech, a member of the Roche Group, in the U.S. and by AbbVie outside of the U.S.
About the MURANO Study
A total of 389 patients with R/R CLL who had received at least one prior therapy were enrolled in the international, multicenter, open-label, randomized (1:1) MURANO study (NCT02005471). The study was designed to evaluate the efficacy and safety of VENCLEXTA in combination with rituximab (194 patients) compared with bendamustine in combination with rituximab (195 patients). The median age of patients in the trial was 65 years (range 22-85).iv
About AbbVie Care
Canadians prescribed VENCLEXTA will have the opportunity to be enrolled in AbbVie Care, AbbVie's signature care program. The program is designed to provide a wide range of customized services including reimbursement and financial support, pharmacy services, lab work reminders and coordination, personalized education and ongoing disease management support throughout the treatment and beyond. For more information, please visit www.abbviecare.ca.
About AbbVie
AbbVie is a global, research and development-driven biopharmaceutical company committed to developing innovative advanced therapies for some of the world's most complex and critical conditions. The company's mission is to use its expertise, dedicated people and unique approach to innovation to markedly improve treatments across four primary therapeutic areas: immunology, oncology, virology and neuroscience. In more than 75 countries, AbbVie employees are working every day to advance health solutions for people around the world. For more information about AbbVie, please visit us at www.abbvie.ca and www.abbvie.com. Follow @abbvieCanada and @abbvie on Twitter or view careers on our Facebook or LinkedIn page.
| i | pan-Canadian Oncology Drug Review (pCODR) Expert Review Committee (pERC) final recommendation. www.cadth.ca/sites/default/files/pcodr/Reviews2019/10162VenetoclaxRituximabCLL_FnRec_approvedbyChair_REDACT_Post_31May2019-final.pdf. Accessed June 2019. |
| ii | Lymphoma Canada. Chronic lymphocytic leukemia. Available at www.lymphoma.ca/lymphoma/lymphoma-101/types-lymphoma/cll. Accessed June 2019. |
| iii | Canadian Cancer Statistics. Chronic lymphocytic leukemia statistics. www.cancer.ca/en/cancer-information/cancer-type/leukemia-chronic-lymphocytic-cll/statistics/?region=on. Accessed June 2019. |
| iv | VENCLEXTA product monograph, AbbVie Corporation. Date of Preparation: September 27, 2016. Date of Revision: February 12, 2019. www.abbvie.ca/content/dam/abbviecorp/ca/en/docs/VENCLEXTA_PM_EN.pdf. Accessed June 2019. |
SOURCE AbbVie
